Current Projects
1.Single-Atom Platinum Catalysts Supported on Well-Defined Ceria Shapes
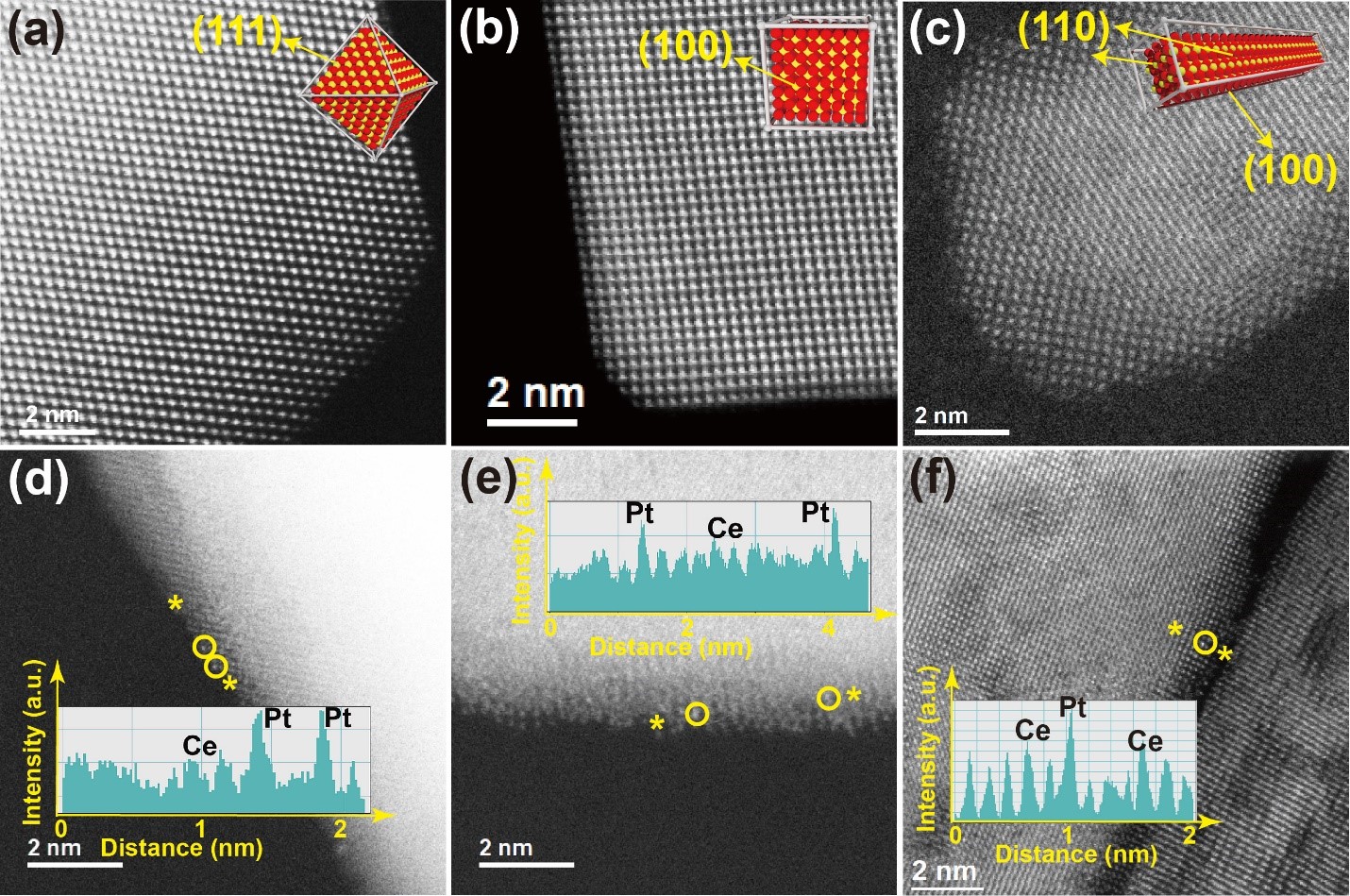
We use a modified Atomic Layer Deposition (ALD) technique (vapor-phase deposition) to synthesize heterogeneous catalysts with atomic-level precision. Ultra-low loadings of active metal (platinum in this project) are utilized to allow synthesis of catalysts with isolated metal atoms or ions. We also utilize well-defined metal oxide nanoparticle shapes as supports, as this allows us to identify metal-support interactions between the isolated metal atoms or ions and the specific surface facets exposed by the various oxide shapes. In this project, we have synthesized three different CeO2 nanoshapes, CeO2 octahedra with (111) surface terminations, CeO2 cubes with (100) surface facets and CeO2 rods with (100) and (110) surface terminations, and the interactions between the isolated Pt and the different CeO2 surface facets have been examined in detail under reducing conditions using various analytical techniques, such as high-angle annular dark field (HAADF) scanning transmission electron microscopy (STEM) and diffuse reflectance infra-red Fourier transform spectroscopy (DRIFTS) using carbon monoxide as a probe molecule (Angew. Chem. Int. Ed. 60 [8] (2021) 4038-4042 or https://doi.org/10.1002/anie.202012469
2.Selective Hydrogenation Reactions
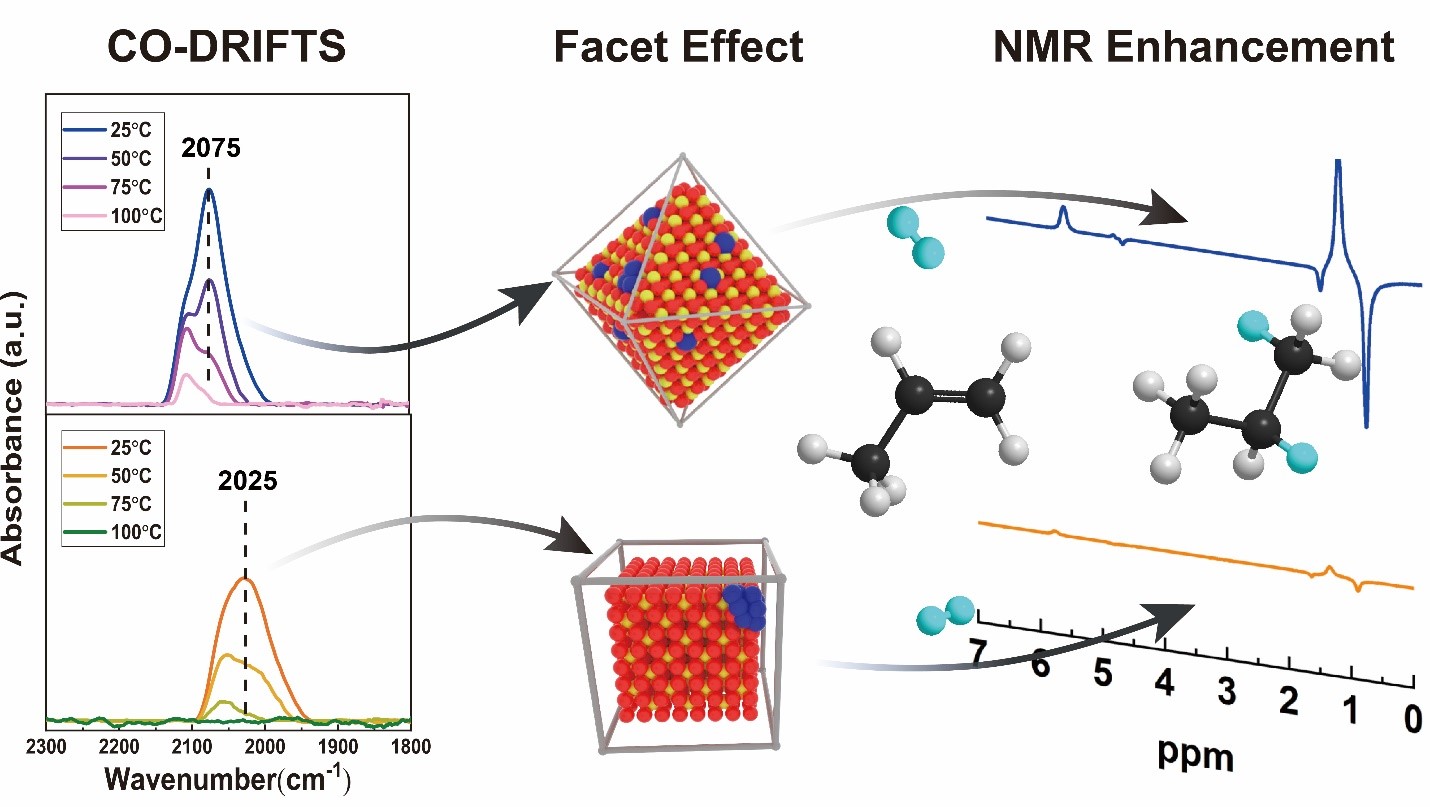
The ultra-low loading Pt catalysts supported on CeO2 shapes have been utilized in the hydrogenation of propene to synthesize spin-labeled propane for parahydrogen-induced polarization (PHIP) nuclear magnetic resonance (NMR). The pair-wise addition of hydrogen to an unsaturated molecule, where both added hydrogen atoms originate from the same parahydrogen molecule (with antiparallel spins), has potential to increase NMR, and thus also magnetic resonance imaging (MRI), signals by four orders of magnitude. We have shown that the electron-deficient Pt species on the (111) surfaces of CeO2 octahedra result in significantly higher pair-wise electivity, and thus also NMR signal enhancement, compared with the electron-poor Pt on the (100) surfaces of CeO2 cubes.
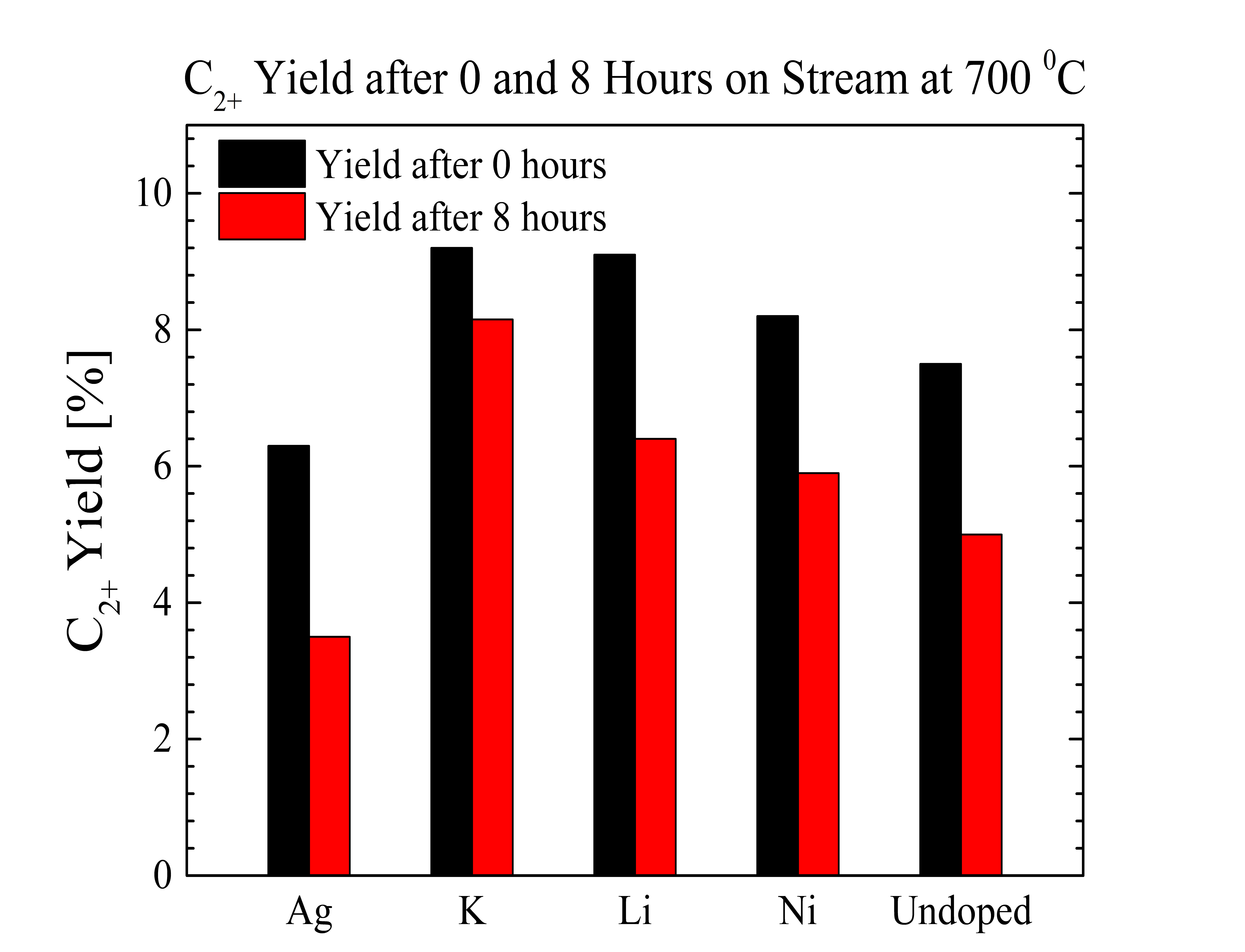
3.Selective Oxidation Reactions – Oxidative coupling of methane
Low-temperature activation of methane and its selective conversion to higher value chemicals is challenging and has been the subject of numerous studies. Our group has focused on the direct conversion of methane to ethane and ethylene over mixed metal oxides and doped rare earth oxides, with specific emphasis on samarium(III) oxide and terbium(IV) oxide. We have shown that reducible rare earth oxides, such as terbium(IV) oxide, which are not expected to be selective to the oxidative coupling of methane, can with the right combination of promoters and dopants be more active and selective than the corresponding non-reducible oxide, such as samarium(III) oxide ().
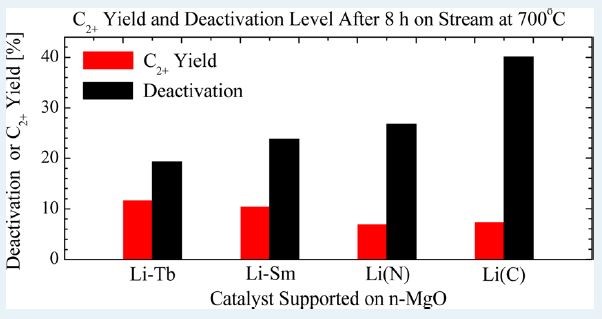
We have also shown that the activity and selectivity of samarium(III) oxide in the oxidative coupling of methane can be improved by the addition of small amounts (0.1 %) of transition metal dopants, even if the oxide of the specific transition metal itself is not expected to be selective to methane coupling. However, the transition metal doped samarium(III) oxides cannot outperform the alkali doped samarium(III) oxides.
4. Advanced Synthesis Strategies
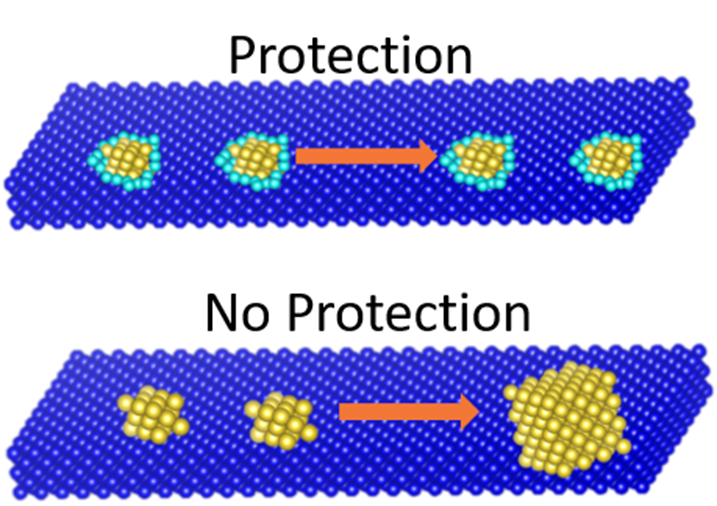
The stability of heterogeneous catalysts consisting of atomically dispersed metals or metal nanoparticles on oxide supports is very important particularly under harsh conditions, such as high temperatures. To improve sintering resistance, an inert protective layer can be deposited over or around the metal particles to reduce the mobility of the metal on the support. The effects of the protective layer and different deposition strategies are being investigated.