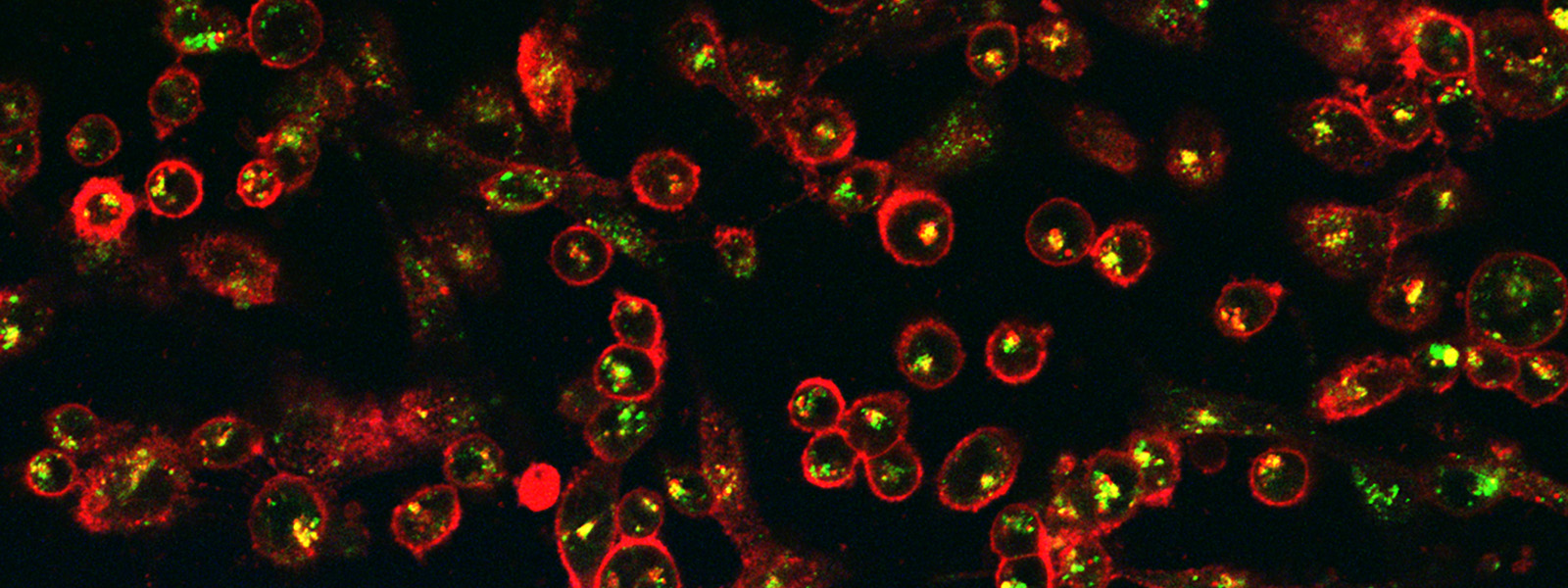
Hyperthermia has been explored as a treatment for disease such as cancer since ancient times. Recently, the development of nanotechnology and realization that iron oxide superparamagnetic nanoparticles can transform the energy of an alternating magnetic field into heat has spurred research in applying nanoscale thermal energy delivery to kill cancer cells. Although initially researchers had imagined that nanoscale heating effects in the vicinity of nanoparticles might be sufficient to damage and kill cells in the absence of a tissue-level temperature rise to the hyperthermia range (43-47°C), the paradigm in the field of nanoparticle thermal cancer therapy since the late 1990s had been that it was impossible to kill cancer cells in this way. Through a combination of magnetic nanoparticle engineering and judicious experimentation, our research has demonstrated that this paradigm was incorrect and that nanoscale heating phenomena in the vicinity of receptor-targeted magnetic nanoparticles can lead to significant (>99%) reductions in cancer cell clonogenic survival without any macroscopic temperature rise. Our work has also demonstrated that one mechanism responsible for cell death is disruption of nanoparticle-loaded lysosomes, activating lysosomal death pathways that are upregulated in many cancer cells. With various collaborators we have studied the combination of magnetic nanoparticle hyperthermia with chemotherapeutics, elucidating underlying mechanisms such as cell membrane permeabilization and enhanced proteotoxic stress. Furthermore, we recently demonstrated the potential of magnetic particle imaging guided magnetic hyperthermia for precise and spatially selective cancer thermal therapy. Our ability to model magnetic nanoparticle behavior in time-varying magnetic fields has allowed us to make fundamental contributions to understanding the roles of non-linear magnetization and bias fields on the energy dissipation rate of magnetic nanoparticles in alternating magnetic fields. More recently, we’ve applied these principles to develop ultra stable magnetic cryopreservation agents that can uniformly perfuse whole organs, eliminate ice crystal formation during vitrification and long term storage in liquid nitrogen, and rapidly and uniformly warm these organs to room temperature avoiding damage to the organ. These findings are transforming the field of magnetic nanoparticle thermal therapy.
Related Publications:
- Andreina Chiu LamG, Edward Staples, Carl Pepine, and Carlos Rinaldi, “Pefusion, cryopreservation, and nanowarming of whole hearts using colloidally stable cryopreservation agent solutions.” Science Advances, 7(2):eabe3005, 2021. [https://doi.org/10.1126/sciadv.abe3005]
- Yao Lu, Angelie Rivera-RodriguezG, Zhi Wei Tay, Daniel Hensley, K.L. Barry Fung, Caylin Colson, Chinmoy Saayujya, Quincy Huynh, Leyla Kabuli, Benjamin Fellows, Prashant Chandrasekharan, Carlos Rinaldi, and Steven Conolly, “Combining magnetic particle imaging and magnetic hyperthermia for localized and image-guided treatment.” International Journal of Hyperthermia, 37(3):141-154, 2020. [https://doi.org/10.1080/02656736.2020.1853252]
- Kacoli SenP, Austin E.F. Scheppe, Ishita SinghG, Winnie Hu, Mariola J. Edelmann, and Carlos Rinaldi, “Exosomes released by breast cancer cells under mild hyperthermic stress possess immunogenic potential and stimulate pro-inflammatory phenotype in vitro in macrophages.” International Journal of Hyperthermia, 37(1):696-710, 2020. [https://doi.org/10.1080/02656736.2020.1778800]
- Prashant Chandrasekharan, Zhi Wei Tay, Daniel Hensley, Xinyi Y Zhou, Barry KL Fung, Caylin Colson, Yao Lu, Benjamin D Fellows, Quincy Huynh, Chinmoy Saayujya, Elaine Yu, Ryan Orendorff, Zheng Bo, Patrick Goodwill, Carlos Rinaldi, and Steven Conolly, “Using magnetic particle imaging systems to localize and guide magnetic hyperthermia treatment: Tracers, hardware, and future medical applications.” Theranostics, 10(7):2965, 2020. [https://doi.org/10.7150/thno.40858]
- Zhiyuan ZhaoG and Carlos Rinaldi, “Magnetization dynamics and energy dissipation of interacting magnetic nanoparticles in alternating magnetic fields with and without a static bias field.” The Journal of Physical Chemistry C, 122(36):21018-21030, 2018. [http://doi.org/10.1021/acs.jpcc.8b04071]
- Zhi Wei Tay, Prashant Chandrasekharan, Andreina Chiu-LamG, Daniel Hensley, Rohan DhavalikarG, Xinyi Zhou, Elaine Yu, Patrick Goodwill, Bo Zheng, Carlos Rinaldi, Steven M. Conolly, “Magnetic Particle Imaging Guided Heating In Vivo using Gradient Fields For Arbitrary Localization of Thermal Therapy.” ACS Nano, 12(4):3699-3713, 2018. [http://doi.org/10.1021/acsnano.8b00893]
- Angelie Rivera-RodriguezG, Andreina Chiu-LamG, Viacheslav Morozov, Alexander Ishov and Carlos Rinaldi, “Magnetic nanoparticle hyperthermia potentiates Paclitaxel activity in sensitive and resistant breast cancer cells.” International Journal of Nanomedicine, 2018(13):4771-4779, 2018. [http://doi.org/10.2147/IJN.S171130]
- Rui Zhang, Benjamin Fellows, Nikorn Pothayee, Nan Hu, Nipon Pothayee, Ami Jo,Ana C. BohorquezG, Carlos Rinaldi, Alan P. Koretsky, O. Thompson Mefford, Rickey M. Davis and Judy S. Riffle, “Ammonium Bisphosphonate-Polymeric Magnetic Nanocomplexes for Platinum Anticancer Drug Delivery and Imaging, with Potential Hyperthermia and Triggered Drug Release.” Journal of Nanomaterials, 2018:4341580, 2018. [http://doi.org/10.1155/2018/4341580]
- Karem A. Court, Hiroto Hatakeyama, Sherry Y. Wu, Mangala S. Lingegowda, Cristian Rodríguez-Aguayo, Gabriel López-Berestein, Lee Ju-Seog, Carlos Rinaldi, Eduardo J. Juan, Anil K. Sood and Madeline Torres-Lugo, “HSP70 inhibition synergistically enhances the effects of magnetic fluid hyperthermia in ovarian cancer.” Molecular Cancer Therapeutics, 16(5):966-976, 2017. [http://doi.org/10.1158/1535-7163.MCT-16-0519]
- Daniel Hensley, Zhi Wei Tay, Rohan DhavalikarG, Bo Zheng, Patrick Goodwill, Carlos Rinaldi, Steven Conolly, “Combining Magnetic Particle Imaging and magnetic fluid hyperthermia in a theranostic platform.” Physics in Medicine and Biology, 62(9):3483, 2017. [http://doi.org/10.1088/1361-6560/aa5601]
- Rohan DhavalikarG and Carlos Rinaldi, “Theoretical Predictions for the Spatial Distribution of Magnetic Nanoparticle Heating in Magnetic Particle Imaging Field Gradients.” Journal of Magnetism and Magnetic Materials, 419:267-273, 2016. [http://doi.org/10.1016/j.jmmm.2016.06.038]
- Andreina Chiu LamG and Carlos Rinaldi, “Nanoscale thermal phenomena in the vicinity of magnetic nanoparticles in alternating magnetic fields.” Advanced Functional Materials, 26(22):3933-3941,2016. [http://doi.org/10.1002/adfm.201505256]
- Fernando Mérida, Andreina ChiuG, Ana BohórquezG, Lorena Maldonado, M.-E. Pérez, L. Pericchi, Madeline Torres-Lugo, Carlos Rinaldi, “Optimization of synthesis and peptization steps to obtain iron oxide nanoparticles with high specific absorption rates.” Journal of Magnetism and Magnetic Materials, 394:361-371,2015. [http://doi.org/10.1016/j.jmmm.2015.06.076]
- Denisse Soto-AquinoG and Carlos Rinaldi, “Nonlinear energy dissipation in magnetic nanoparticle suspensions,” Journal of Magnetism and Magnetic Materials, 393:46-55,2015. [http://doi.org/10.1016/j.jmmm.2015.05.009]
- Merlis Alvarez-Berrios, Angel Castillo, Carlos Rinaldi, and Madeline Torres-Lugo, “Enhanced proteotoxic stress: one of the contributors for hyperthermic potentiation of the proteasome inhibitor bortezomib using magnetic nanoparticles,” Biomaterials Science, 3:391-400,2015. [http://doi.org/10.1039/c4bm00223g]
- Nicole IovinoM, Ana C. BohorquezG, and Carlos Rinaldi, “Magnetic nanoparticle targeting of lysosomes: a viable method of overcoming tumor resistance?” Nanomedicine, 9(7):937-939, 2014. [http://doi.org/10.2217/NNM.14.52]
- Merlis Alvarez-Berrios, Angel Castillo, Carlos Rinaldi, and Madeline Torres-Lugo, “Magnetic fluid hyperthermia enhances cytotoxicity of bortezomib in sensitive and resistant cancer cells lines,.” International Journal of Nanomedicine, 9:145-153, 2014. [http://doi.org/10.2147/IJN.S51435]
- G.A. Sotiriou, M.A. Visbal-Onufrak, A. Teleki, Eduardo J. Juan, A.M. Hirt, S.E. Pratsinis, and Carlos Rinaldi, “Thermal energy dissipation by SiO2-coated plasmonic superparamagnetic nanoparticles in alternating magnetic fields.” Chemistry of Materials, 25(22):4603-4612, 2013. [http://doi.org/10.1021/cm402896x]
- Roberto Olayo-VallesG and Carlos Rinaldi, “Modulation of interparticle interactions and specific absorption rate in magnetomicelles through changes in molecular weight of hydrophobic polymer block.” Particle & Particle System Characterization, 30(11):964-971, 2013. [http://doi.org/10.1002/ppsc.201300157]
- B. Kozissnik, Ana C. BohorquezG, Jon Dobson, and Carlos Rinaldi, “Magnetic Fluid Hyperthermia: Advances, Challenges, and Opportunities.” International Journal of Hyperthermia, 29(8):706-714, 2013.[http://doi.org/10.3109/02656736.2013.837200]
- Madeline Torres-Lugo and Carlos Rinaldi, “Thermal Potentiation of Chemotherapeutics by Inorganic Nanoparticles.” Nanomedicine, 8(10):1689-1707, 2013. [http://doi.org/10.2217/NNM.13.146]
- Maribella DomenechP, Ileana Marrero-BerriosU, Madeline Torres-Lugo, and Carlos Rinaldi, “Lysosomal Membrane Permeabilization by Targeted Magnetic Nanoparticles in Alternating Magnetic Fields.” ACS Nano, 7(6):5091-5101, 2013. [http://doi.org/10.1021/nn4007048]
- Merlis Alvarez-Berrios, Angel Castillo, Janet Mendéz, Oscar Soto, Carlos Rinaldi, and Madeline Torres-Lugo, “Hyperthermic potentiation of cisplatin by magnetic nanoparticle heaters is correlated with an increase in cell membrane fluidity.” International Journal of Nanomedicine, 2013(8): 1003-1013, 2013. [http://doi.org/10.2147/IJN.S38842]
- Liliana Polo-CorralesG and Carlos Rinaldi, “Monitoring iron oxide nanoparticle surface temperature in an alternating magnetic field using thermoresponsive-fluorescent polymers.” Journal of Applied Physics, 111:07B334, 2012. [http://doi.org/10.1063/1.3680532]
- Mar Creixell,G Ana C. Bohorquez, Madeline Torres-Lugo, and Carlos Rinaldi, “EGFR-targeted magnetic nanoparticle heaters can kill cancer cells without a perceptible temperature rise.” ACS Nano, 5(9), 7124-7129, 2011. [http://doi.org/10.1021/nn201822b]
- Jason S. Lee, Hector L. Rodríguez-Luccioni, Anil K. Sood, Gabriel Lopez-Berestein, Carlos Rinaldi, and Madeline Torres-Lugo, “Hyperthermia induced by magnetic nanoparticles improves the effectiveness of the anticancer drug cis-diamminedichloroplatinum.” Journal of Nanoscience and Nanotechnology, 11:4153-4157, 2011. [http://doi.org/10.1166/jnn.2011.3821]
- Hector L.Rodriguez-Luccioni, Magda Latorre-EstevesP, J. Mendez, Oscar Soto, A.R. Rodríguez, Carlos Rinaldi, and Madeline Torres-Lugo, “Enhanced reduction in cell viability due to magnetic fluid hyperthermia compared to hyperthermia using a water bath,.” International Journal of Nanomedicine, 6:373-380, 2011. [http://doi.org/10.2147/IJN.S14613].
- M. Giordano, G. Gutierrez, and Carlos Rinaldi, “Fundamental solutions to the bioheat equation and their application to magnetic fluid hyperthermia.” International Journal of Hyperthermia, 26(5):475-484, 2010. [http://doi.org/10.3109/02656731003749643]
- Magda Latorre and Carlos Rinaldi, “Applications of Magnetic Nanoparticles in Medicine: Magnetic Fluid Hyperthermia.” Puerto Rico Health Sciences Journal, 28(3): 227-238,September 2009.